Chemical methods for protein site-specific ubiquitination
Abstract
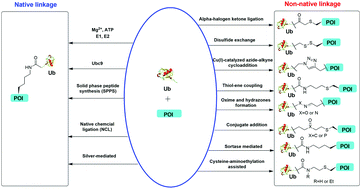
Ubiquitination is an important protein post-translational modification regulating many cellular processes in eukaryotes. Ubiquitination is catalyzed by a three-enzyme cascade resulting in the conjugation of the C-terminal carboxylate of ubiquitin (Ub) to the ε-amino group of a lysine residue in the acceptor protein via an isopeptide bond. In vitro enzymatic ubiquitination utilizing Ub ligases has been successfully employed to generate Ub dimers and polymers. However, limitations of the enzymatic approach exist, particularly due to the requirement of specific Ub ligase for any given target protein and the low catalytic efficiency of the Ub ligase. To achieve an in-depth understanding of the molecular mechanism of Ub signaling, new methods are needed to generate mono- and poly-ubiquitinated proteins at a specific site with defined polyubiquitin chain linkage and length. Chemical methods offer an attractive solution to the above-described challenges. In this review, we summarize the recently developed chemical methods for generating ubiquitinated proteins using synthetic and semisynthetic approaches. These new tools and approaches, as an important part of the Ub toolbox, are crucial to our understanding and exploitation of the Ub system for novel therapeutics.
- This article is part of the themed collection: The Chemical Biology of Peptides


 Please wait while we load your content...
Please wait while we load your content...