Natural Trojan horse inhibitors of aminoacyl-tRNA synthetases†
Abstract
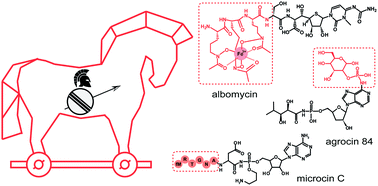
For most antimicrobial compounds with intracellular targets, getting inside the cell is the major obstacle limiting their activity. To pass this barrier some antibiotics mimic the compounds of specific interest for the microbe (siderophores, peptides, carbohydrates, etc.) and hijack the transport systems involved in their active uptake followed by the release of a toxic warhead inside the cell. In this review, we summarize the information about the structures, biosynthesis, and transport of natural inhibitors of aminoacyl-tRNA synthetases (albomycin, microcin C-related compounds, and agrocin 84) that rely on such “Trojan horse” strategy to enter the cell. In addition, we provide new data on the composition and distribution of biosynthetic gene clusters reminiscent of those coding for known Trojan horse aminoacyl-tRNA synthetases inhibitors. The products of these clusters are likely new antimicrobials that warrant further investigation.
- This article is part of the themed collection: Small molecules for chemical biology


 Please wait while we load your content...
Please wait while we load your content...