Solution-based chemical pre-alkaliation of metal-ion battery cathode materials for increased capacity†
Abstract
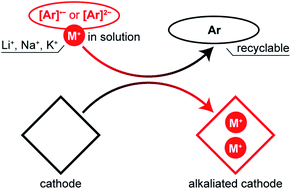
For metal-ion batteries, the limited amount of metal ions that can be reversibly extracted from a cathode is a major problem, which leads to decreased reversible capacity (mA h g−1) and energy density (W h g−1). For a great number of cathode materials with attractive properties, it is impossible to enable their full capacity without alkaliated anodes, which are often highly reactive. Having a reliable and scalable approach for cathode pre-alkaliation, i.e., addition of extractable alkali metal ions prior to the battery assembling, should be helpful for the practical implementation of these emerging materials. Cathode treatment with solutions of alkali metal salts derived from aromatic molecules that undergo reversible reduction is an attractive approach for this purpose. Here we show that this approach is applicable for the pre-alkaliation of various cathode materials, both organic and inorganic, which can be used for lithium-, sodium- or potassium-ion batteries. Several types of reducing agents, derived from arenes (exemplified by naphthalene) or heterocyclic compounds (exemplified by phenazine), were shown to be suitable, and the pre-alkaliation degree could be controlled by varying the reducing agent nature or its amount.


 Please wait while we load your content...
Please wait while we load your content...