Rational design of Ti-based oxygen redox layered oxides for advanced sodium-ion batteries†
Abstract
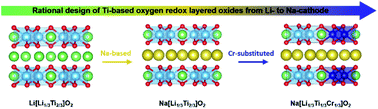
Considering Mn4+ (3d3)-based cations, various layered oxides (A[AyM1−y]O2, where A and M refer to alkali metals and transition metals, respectively) exhibiting oxygen-redox reactions have been investigated extensively to achieve high energy density in lithium-ion batteries (LIBs) and sodium-ion batteries (SIBs). Because M redox activity is determined by the energetics of crystal field theory for oxides, we systematically design a Ti4+ (3d0)-based Na layered oxide, i.e., Na[Li1/3Ti1/3Cr1/3]O2, which features a cation–anion-coupled redox reaction based on the following four rational steps. First, we demonstrate a Ti-based Li layered oxide (Li[Li1/3Ti2/3]O2) undergoing deintercalation at an extremely high voltage to deliver immense oxygen redox capacities for LIBs. Second, we rationally design a Na[Li1/3Ti2/3]O2 layered oxide that shows a high-voltage trend at ≈4.4 V vs. Na+/Na with a high capacity of ≈300 mA h g−1. Third, it is unambiguous that unhybridized O 2p-electrons are vital for compensating the charge imbalance induced by Na removal because of the rigid Ti4+ electronic structure. Fourth, two types of cations are incorporated into the M layer, i.e., redox-inactive (Ti) and redox-active (Cr) cations. Na[Li1/3Ti1/3Cr1/3]O2 is considered superior as it exhibits phase stability when utilizing the oxygen-redox activity and maintains the initial charge character upon cycling.


 Please wait while we load your content...
Please wait while we load your content...