Detailed redox mechanism and self-discharge diagnostic of 4.9 V LiMn1.5Ni0.5O4 spinel cathode revealed by Raman spectroscopy†
Abstract
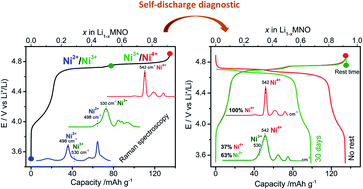
Lithium-ion batteries are commonly used for electrical energy storage in portable devices and are promising systems for large-scale energy storage. However, their application is still limited due to electrode degradation and stability issues. To enhance the fundamental understanding of electrode degradation, we report on the Raman spectroscopic characterization of the promising 5 V spinel LiMn1.5Ni0.5O4 (LMNO) composite cathode. The Raman spectral variations displayed during the charge–discharge cycle in the 3.5–4.9 V vs. Li+/Li potential range are shown to be strongly associated with the change in the transition metals valence states. A careful electrochemical and spectroscopic analysis allows identifying specific descriptors of the Ni2+/Ni3+/Ni4+ species in the Raman spectra and providing their relative ratio during the redox process. This combined approach demonstrates the efficiency of Raman spectroscopy to determine the state of charge (SOC) of the LMNO cathode, paving the way for a fast and reliable measure of the self-discharge phenomenon in the spinel electrode.


 Please wait while we load your content...
Please wait while we load your content...