Unprecedented electrocatalytic oxygen evolution performances by cobalt-incorporated molybdenum carbide microflowers with controlled charge re-distribution†
Abstract
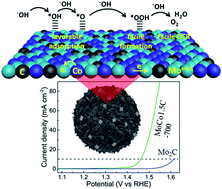
Molybdenum carbide (MC) is a highly active electrocatalyst for the hydrogen evolution reaction (HER) due to its Pt-like d-band electronic density of states. Although a significant improvement in the HER activity was observed, much less effort has been made to realize the oxygen evolution reaction (OER) properties from MC. In this work, we propose controlled charge transfer from cobalt to MC, leading to an unprecedented OER performance. Electron transfer from Co to Mo was established by X-ray photoelectron spectroscopy (XPS) and X-ray absorption near-edge spectroscopy (XANES) analyses. The optimized electrocatalyst, MoCo1.5C-700, showed a low overpotential of 232.5 mV to achieve a current density of 10 mA cm−2 and a small Tafel slope of 61 mV dec−1 for the OER in an alkaline medium. The electrocatalyst exhibited excellent durability for over 27 h at a current density of 10 mA cm−2. Density functional theory (DFT) calculation proved favorable adsorption of the OER intermediates (*OH and *O) over Co-incorporated MC that facilitated the formation of *OOH, which led to an accelerated O2 evolution. In addition, the electrocatalyst displayed superb HER performance, which was confirmed by an overpotential of 96.5 mV to attain a current density of 10 mA cm−2 in 1.0 M KOH. We anticipate that our work will open up new ways to develop efficient and noble metal-free electrocatalysts for overall water splitting.


 Please wait while we load your content...
Please wait while we load your content...