Efficient synthesis of antiviral agent uprifosbuvir enabled by new synthetic methods†
Abstract
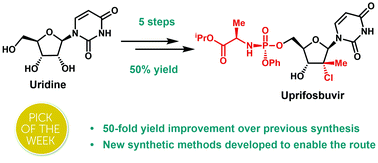
An efficient route to the HCV antiviral agent uprifosbuvir was developed in 5 steps from readily available uridine in 50% overall yield. This concise synthesis was achieved by development of several synthetic methods: (1) complexation-driven selective acyl migration/oxidation; (2) BSA-mediated cyclization to anhydrouridine; (3) hydrochlorination using FeCl3/TMDSO; (4) dynamic stereoselective phosphoramidation using a chiral nucleophilic catalyst. The new route improves the yield of uprifosbuvir 50-fold over the previous manufacturing process and expands the tool set available for synthesis of antiviral nucleotides.
- This article is part of the themed collections: 2021 ChemSci Pick of the Week Collection and 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...