The underlying mechanism for reduction stability of organic electrolytes in lithium secondary batteries†
Abstract
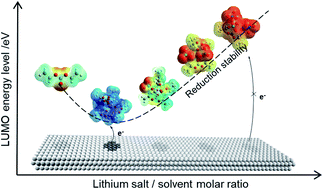
Many organic solvents have very desirable solution properties, such as wide temperature range, high solubility of Li salts and nonflammability, and should be able but fail in reality to serve as electrolyte solvents for Li-ion or -metal batteries due to their reduction instability. The origin of this interfacial instability remains unsolved and disputed so far. Here, we reveal for the first time the origin of the reduction stability of organic carbonate electrolytes by combining ab initio molecular dynamics (AIMD) simulations, density functional theory (DFT) calculations and electrochemical stability experiments. It is found that with the increase of the molar ratio (MR) of salt to solvent, the anion progressively enters into the solvation shell of Li+ to form an anion-induced ion–solvent-coordinated (AI-ISC) structure, leading to a “V-shaped” change of the LUMO energy level of coordinated solvent molecules, whose interfacial stability first decreases and then increases with the increased MRs of salt to solvent. This mechanism perfectly explains the long-standing puzzle about the interfacial compatibility of organic electrolytes with Li or similar low potential anodes and provides a basic understanding and new insights into the rational design of the advanced electrolytes for next generation lithium secondary batteries.
- This article is part of the themed collection: 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...