An umpolung-enabled copper-catalysed regioselective hydroamination approach to α-amino acids†
Abstract
A copper-catalysed regio- and stereoselective hydroamination of acrylates with hydrosilanes and hydroxylamines has been developed to afford the corresponding α-amino acids in good yields. The key to regioselectivity control is the use of hydroxylamine as an umpolung, electrophilic amination reagent. Additionally, a judicious choice of conditions involving the CsOPiv base and DTBM-dppbz ligand of remote steric hindrance enables the otherwise challenging C–N bond formation at the α position to the carbonyl. The point chirality at the β-position is successfully controlled by the Xyl-BINAP or DTBM-SEGPHOS chiral ligand with similarly remote steric bulkiness. The combination with the chiral auxiliary, (−)-8-phenylmenthol, also induces stereoselectivity at the α-position to form the optically active unnatural α-amino acids with two adjacent stereocentres.
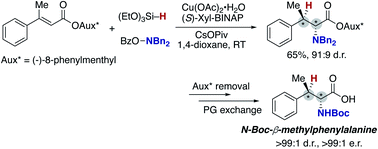


 Please wait while we load your content...
Please wait while we load your content...