CO/light dual-activatable Ru(ii)-conjugated oligomer agent for lysosome-targeted multimodal cancer therapeutics†
Abstract
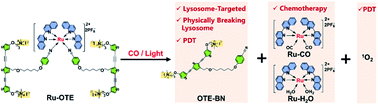
Stimuli-activatable and subcellular organelle-targeted agents with multimodal therapeutics are urgently desired for highly precise and effective cancer treatment. Herein, a CO/light dual-activatable Ru(II)-oligo-(thiophene ethynylene) (Ru-OTE) for lysosome-targeted cancer therapy is reported. Ru-OTE is prepared via the coordination-driven self-assembly of a cationic conjugated oligomer (OTE-BN) ligand and a Ru(II) center. Upon the dual-triggering of internal gaseous signaling molecular CO and external light, Ru-OTE undergoes ligand substitution and releases OTE-BN followed by dramatic fluorescence recovery, which could be used for monitoring drug delivery and imaging guided anticancer treatments. The released OTE-BN selectively accumulates in lysosomes, physically breaking their integrity. Then, the generated cytotoxic singlet oxygen (1O2) causes severe lysosome damage, thus leading to cancer cell death via photodynamic therapy (PDT). Meanwhile, the release of the Ru(II) core also suppresses cancer cell growth as an anticancer metal drug. Its significant anticancer effect is realized via the multimodal therapeutics of physical disruption/PDT/chemotherapy. Importantly, Ru-OTE can be directly photo-activated using a two-photon laser (800 nm) for efficient drug release and near-infrared PDT. Furthermore, Ru-OTE with light irradiation inhibits tumor growth in an MDA-MB-231 breast tumor model with negligible side effects. This study demonstrates that the development of an activatable Ru(II)-conjugated oligomer potential drug provides a new strategy for effective subcellular organelle-targeted multimodal cancer therapeutics.


 Please wait while we load your content...
Please wait while we load your content...