Mechanochemical activation of disulfide-based multifunctional polymers for theranostic drug release†
Abstract
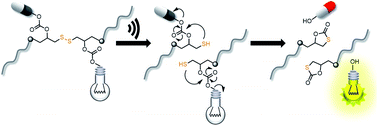
Drug delivery systems responsive to physicochemical stimuli allow spatiotemporal control over drug activity to overcome limitations of systemic drug administration. Alongside, the non-invasive real-time tracking of drug release and uptake remains challenging as pharmacophore and reporter function are rarely unified within one molecule. Here, we present an ultrasound-responsive release system based on the mechanochemically induced 5-exo-trig cyclization upon scission of disulfides bearing cargo molecules attached via β-carbonate linker within the center of a water soluble polymer. In this bifunctional theranostic approach, we release one reporter molecule per drug molecule to quantitatively track drug release and distribution within the cell in real-time. We use N-butyl-4-hydroxy-1,8-naphthalimide and umbelliferone as fluorescent reporter molecules to accompany the release of camptothecin and gemcitabine as clinically employed anticancer agents. The generality of this approach paves the way for the theranostic release of a variety of probes and drugs by ultrasound.


 Please wait while we load your content...
Please wait while we load your content...