Enantioselective synthesis of chiral porphyrin macrocyclic hosts and kinetic enantiorecognition of viologen guests†
Abstract
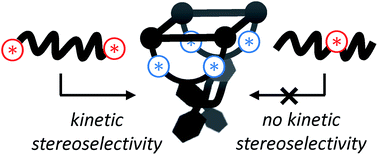
The construction of macromolecular hosts that are able to thread chiral guests in a stereoselective fashion is a big challenge. We herein describe the asymmetric synthesis of two enantiomeric C2-symmetric porphyrin macrocyclic hosts that thread and bind different viologen guests. Time-resolved fluorescence studies show that these hosts display a factor 3 kinetic preference (ΔΔG‡on = 3 kJ mol−1) for threading onto the different enantiomers of a viologen guest appended with bulky chiral 1-phenylethoxy termini. A smaller kinetic selectivity (ΔΔG‡on = 1 kJ mol−1) is observed for viologens equipped with small chiral sec-butoxy termini. Kinetic selectivity is absent when the C2-symmetric hosts are threaded onto chiral viologens appended with chiral tails in which the chiral moieties are located in the centers of the chains, rather than at the chain termini. The reason is that the termini of the latter guests, which engage in the initial stages of the threading process (entron effect), cannot discriminate because they are achiral, in contrast to the chiral termini of the former guests. Finally, our experiments show that the threading and de-threading rates are balanced in such a way that the observed binding constants are highly similar for all the investigated host–guest complexes, i.e. there is no thermodynamic selectivity.


 Please wait while we load your content...
Please wait while we load your content...