Effect of monovalent salt concentration and peptide secondary structure in peptide-micelle binding†
Abstract
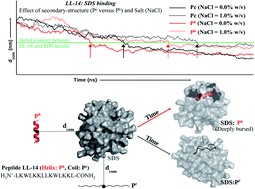
Recently, we reported a cationic 14 residue peptide LL-14 (LKWLKKLLKWLKKL) with salt-sensitive broad-spectrum antimicrobial potency. However, the mechanism of its salt (NaCl) sensitivity remained unclear. In this study, we have reported computational (∼14.2 μs of MD) and experimental (CD, fluorescence) investigations to examine the salt-sensitivity and the role of peptide secondary structure on LL-14 binding to simple membrane mimetic (SDS, DPC) systems. LL-14 was shown to adopt a random coil (Pc) conformation in water and α-helical conformation (Ph) in the peptide:SDS micelle complex, accompanied by tryptophan burial, using both simulations and experiments. Simulations successfully deconvoluted the LL-14:micelle binding event in terms of secondary structure (random coil Pc versus helix Ph) and gave atomic insight into the initial and final LL-14:SDS complexes. Electrostatics drove the N-terminus (L1 and K2) of LL-14 (Pc or Ph) to bind the SDS micellar surface, initiating complex formation. LL-14 in amphipathic Ph conformation bound faster and buried deeper into the SDS micelle relative to Pc. Increasing NaCl concentration incrementally delayed LL-14:micelle binding by shielding the overall charges of the interacting partners. LL-14 binding to the SDS micelle was significantly faster relative to that of the zwitterionic DPC micelle due to electrostatic reasons. Cationic α-helical amphipathic peptides (with positively charged N-terminus) with low salt-ion concentration seemed to be ideal for faster SDS binding.


 Please wait while we load your content...
Please wait while we load your content...