One-pot synthesis of spherical nanoscale zero-valent iron/biochar composites for efficient removal of Pb(ii)
Abstract
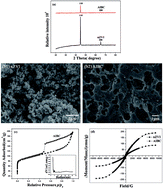
In this study, a spherical Fe/C composite (AIBC) was successfully prepared via carbonization of Fe3+-crosslinked sodium alginate. The removal capacity and mechanism of AIBC were evaluated for the adsorption of Pb(II) from aqueous solution and compared with that of commercial nanoscale zero-valent iron (nZVI). The effects of the initial concentration, pH of Pb(II) solution, the contact time, coexisting anions, and aging under air were investigated. The results showed that the pH had a strong impact on the adsorption of Pb(II) by AIBC. The adsorption data for AIBC followed the Langmuir model, while the maximum adsorption capacity at pH 5 was 1881.73 mg g−1. The AIBC had a higher adsorption capability than nZVI, especially under the condition of relatively high Pb(II) concentrations. The oxidation–reduction reaction between Fe and Pb(II) was the main mechanism for the adsorption of Pb(II) onto nZVI. AIBC converted the largest amount of Pb(II) into PbO·XH2O/Pb(OH)2 mainly by generating Fe2+.


 Please wait while we load your content...
Please wait while we load your content...