Long-wavelength visible to near infrared photoluminescence from carbon-bridged styrylstilbene and thiadiazole conjugates in organic and aqueous media†
Abstract
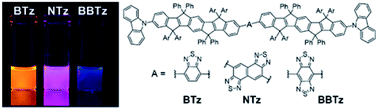
Donor–acceptor–donor conjugates composed of electron-donating carbon-bridged styrylstilbene (COPV2) and electron-accepting thiadiazole derivatives equipped with carbazolyl (Cz) terminators, Cz-COPV2-A-COPV2-Cz (A = benzothiadiazole (BTz), naphthobis(thiadiazole) (NTz), or benzobis(thiadiazole) (BBTz)), were newly synthesized and found to serve as efficient and stable long-wavelength photoluminescent dyes in organic and aqueous media. In particular, Cz-COPV2-BBTz-COPV2-Cz showed photoluminescence in the near infrared region (895–927 nm) with a photoluminescence quantum yield (PLQY) of up to 0.19 in cyclohexane and of 0.02–0.03 in THF/water mixtures. Its analogues with weaker acceptors, Cz-COPV2-BTz-COPV2-Cz and Cz-COPV2-NTz-COPV2-Cz, showed yellow to deep-red emission in organic solvents, with PLQYs of up to 0.71 in organic solvents and 0.45 in THF/water mixtures.


 Please wait while we load your content...
Please wait while we load your content...