Regio- and enantioselective amination of acyclic branched α-alkynyl ketones: asymmetric construction of N-containing quaternary stereocenters†
Abstract
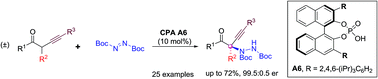
The direct regio- and enantioselective amination of acyclic branched α-alkynyl ketones with azodicarboxylates has been developed through chiral phosphoric acid catalysis, which generates α-hydrazido-α-alkynyl ketone products with high enantioselectivity. Control experiments indicate that the α-alkynyl group in the ketone substrate is critical for both the reactivity and stereoselectivity of the reaction. Facile derivatizations of the functional group-abundant chiral product further highlight the value of this approach in the asymmetric synthesis of α-tertiary amines and N-containing heterocycles.


 Please wait while we load your content...
Please wait while we load your content...