The dehydration of N-acetylglucosamine (GlcNAc) to enantiopure dihydroxyethyl acetamidofuran (Di-HAF)†
Abstract
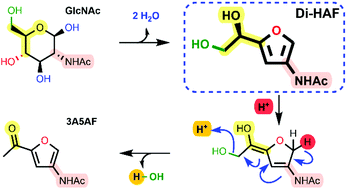
The first multi-gram synthesis of enantiopure dihydroxyethyl acetamidofuran (Di-HAF) is reported. Under optimized conditions, GlcNAc dehydrates in pyridine in the presence of phenylboronic acid and triflic acid to afford Di-HAF in 73% yield and 99.3% ee in just 30 minutes. This protocol opens the door for further research on this bio-renewable building block which is now available as a chiral pool synthon. A plausible mechanism of its formation and of the subsequent dehydration of Di-HAF into well-known 3-acetamido-5-acetylfuran (3A5AF) is proposed.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...