Pd-Catalyzed C(sp2)–H olefination: synthesis of N-alkylated isoindolinone scaffolds from aryl amides of amino acid esters†
Abstract
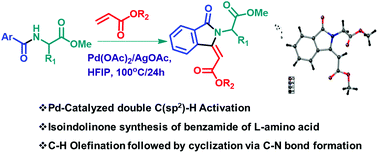
Isoindolinone is a constituent of various natural products and synthetic biologically active compounds. The classical multi-step synthetic methods used to prepare various indolinone derivatives are tedious and challenging. One-pot synthetic methods are attractive and economical. Transition-metal-catalyzed C–H activation is an emerging tool for synthesizing natural products and small organic molecules via reducing the number of synthetic steps necessary. This paper describes the synthesis of N-alkyl-3-methenyl chiral isoindolinone derivatives from aryl amides of L-amino acids and non-activated alkene via Pd-catalyzed C(sp2)–H olefination. Herein, the amino acid residue acts as a directing group for olefination at the aryl ring, and then cyclization occurs at the amide NH. Hence, this methodology could be helpful to transform standard amino acids into respective chiral isoindolinone derivatives.


 Please wait while we load your content...
Please wait while we load your content...