Stereodivergent Pd/Cu catalysis: asymmetric alkylation of racemic symmetrical 1,3-diphenyl allyl acetates†
Abstract
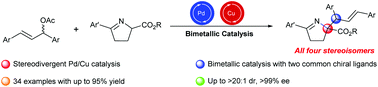
We report a stereodivergent Pd/Cu catalyst system that was successfully applied to the asymmetric allylic alkylation of symmetrical 1,3-disubstituted allyl acetates with prochiral imino esters, providing efficient access to enantiopure products bearing vicinal stereocenters in a fully stereodivergent manner. The protocol proceeds smoothly under mild reaction conditions and can accommodate a range of imino esters, delivering the substituted products in high yields and with excellent diastereoselectivities (up to >20 : 1 dr) and enantioselectivities (up to >99% ee).
- This article is part of the themed collection: Hybrid Catalysis


 Please wait while we load your content...
Please wait while we load your content...