Protecting-group-free S-glycosylation towards thioglycosides and thioglycopeptides in water†
Abstract
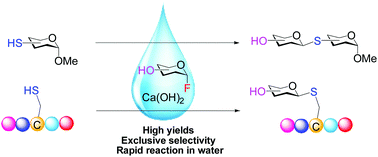
A facile and green S-glycosylation method has been developed featuring protecting-group-free and proceeding-in-water like enzymatic synthesis. Glycosylation of fluoride donors with thiol sugar acceptors using Ca(OH)2 as a promoter afforded various thioglycosides in good yields with exclusive stereoselectivity. This method also enabled the successful production of S-linked oligosaccharides and S-linked glycopeptides.
- This article is part of the themed collection: 2021 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...