Generation and reactivity of an elusive base-stabilised phosphinidene†
Abstract
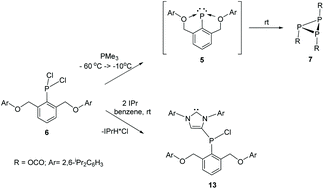
Reduction of phosphorus dichloride 6, supported by the diaryloxyphenyl group (OCO) featuring two bulky phenoxy wingtips, by PMe3, generates a reactive intermediate that behaves as a base-stabilized phosphinidene (OCO)P (5). Warming up a solution of this species in toluene to room temperature results in trimerization to give the isolable cyclic triphosphine [(OCO)P]3, whereas in situ trapping with 2,3-dimethylbutadiene-1,3 afforded a 3,4-dimethylphospholene-3. Investigation of the reduction of 6 by the phosphine PMe3 by NMR led to the observation of a persistent species between −10 °C and 10 °C. A DFT study of this process suggests that this compound cannot be the proposed phosphinidene 5, and is more likely the diphosphine (OCO)ClP-PCl(OCO) (12). Attempted reduction of 5 by the bulky carbene IPr resulted in unusual electrophilic substitution in the carbene olefin backbone by the chlorophosphinyl group.


 Please wait while we load your content...
Please wait while we load your content...