Design, synthesis, structural, spectral, and redox properties and phenoxazinone synthase activity of tripodal pentacoordinate Mn(ii) complexes with impressive turnover numbers†
Abstract
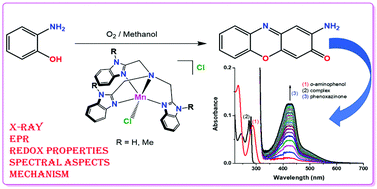
Catechol oxidase (CO) and phenoxazinone synthase (PHS) are two enzymes of immense significance due to their capability to oxidize catechols and o-aminophenols to o-quinones and phenoxazinones, respectively. In this connection two mononuclear manganese complexes with the molecular framework [MnII(Ln)Cl]Cl {L1: tris((1H-benzo[d]imidazol-2-yl)methyl)amine; n = 1 and L2: tris(N-methylbenzimidazol-2-ylmethyl)amine; n = 2} have been designed to be potential catalysts for OAPH (o-aminophenol) oxidation. Both the ligands and their corresponding metal complexes have been successfully synthesized and thoroughly characterized by different spectroscopic and analytical techniques such as FT-IR, 1H NMR, UV-vis spectroscopy, EPR spectroscopy and ESI mass spectroscopy. The molecular structures of [MnII(L1)Cl]Cl (1) and [MnII(L2)Cl]Cl (2) have been revealed by a single-crystal X-ray diffraction study. The spectral properties and redox behaviour of both the complexes were examined. Under ambient conditions, 1 and 2 show excellent phenoxazinone synthase activity as both are very susceptible to oxidize o-aminophenol to phenoxazinone. The kinetic parameters for both complexes have been determined by analyzing the experimental spectroscopic data. The turnover numbers (kcat value) of these two complexes are extremely high, 440 h−1 and 234 h−1 for 1 and 2, respectively. The present report offers a thorough overview of information involving the role of the metal ions and their extent of phenoxazinone synthase mimicking activity. The oxidation of o-aminophenol to 2-amino-3H-phenoxazine-3-one (APX) by catalytic oxidation of oxygen (O2) by the reaction with transition metal complexes has been an important study for the last few decades. The current study evidently showed better performance of our synthesized Mn(II) complexes than all the predecessors. The plausible mechanism has been reiterated based on the experimental data via ESI-MS spectra and considering the concepts from the previously reported mechanisms involved in the formation of hydrogen peroxide (H2O2) as an intermediate substrate is fairly indicating the involvement of molecular oxygen in the catalytic cycle.


 Please wait while we load your content...
Please wait while we load your content...