Theoretical investigation of CH-bond activation by photocatalytic excited SO2 and the effects of C-, N-, S-, and Se-doped TiO2†
Abstract
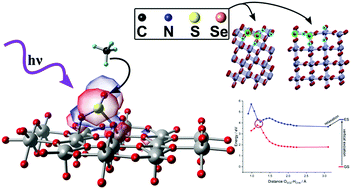
The photocatalytic sulfoxidation on TiO2 discovered by Parrino et al. represents a new, interesting and lower energy route for the synthesis of sulfonic acids. Sulfonic acids are important precursors for dyes, detergents and drugs. In the commonly known industrial process, SO2 and a specific hydrocarbon are converted into sulfonic acids using high-energy UV light. In this reaction, SO2 is excited into a metastable triplet state (3SO2), which has the potential to activate a CH-bond of hydrocarbons and start a radical reaction cycle. By introducing TiO2 as a photocatalyst, it has been shown that visible light can be used for the synthesis. This offers the potential to be a cost-effective reaction approach for industrial use. However, experimental studies indicate that the initial excitation mechanism of SO2 on TiO2 is significantly different from the catalyst-free mechanism. Parrino et al. were able to reveal first evidence for the existence of a charge-transfer process from SO2 to the TiO2 surface by means of electrochemical experiments. First theoretical investigations from first principles were able to further substantiate the existence of a charge-transfer. However, to fully understand this mechanism, more accurate methods such as Time Dependent Density Functional Theory (TD-DFT) or ab initio multireference methods such as the Complete Active Space Self Consistent Field (CASSCF) method are required. Furthermore, after understanding the charge-transfer mechanism, the introduction of dopants into TiO2 can be investigated in order to possibly redshift the excitation energy. This might open the route to using lower energy light for the sulfoxidation of hydrocarbons on TiO2 as a new potential industrial reaction for the synthesis of sulfonic acids. In this work, we will study the initial step of the photocatalytic sulfoxidation of hydrocarbons using the TD-DFT and CASSCF methods by using a combined approach consisting of calculations with periodic boundary conditions and a newly constructed embedded cluster model. Furthermore, we will explore the effects of doping by introducing four heteroatoms (C, N, S, and Se) into the TiO2 surfaces anatase[101] and rutile[110] to find a possible enhancement of the photocatalytic reactivity by lowering the electronic excitation energy.
- This article is part of the themed collection: 2022 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...