Enantioselective construction of a tetrasubstituted stereocenter in isoindolinones via an organocatalyzed reaction between ketones and 3-hydroxyisoindolinones†
Abstract
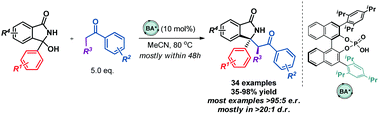
An efficient enantioselective reaction between ketones and 3-hydroxyisoindolinones is described. In a reaction catalyzed by a chiral phosphoric acid, a broad range of ketones and in situ generated ketimines afforded isoindolinone derivatives comprising a tetrasubstituted stereocenter in high yields and enantioselectivities. The developed methodology is also suitable for the construction of compounds with vicinal stereogenic centers.


 Please wait while we load your content...
Please wait while we load your content...