Iridium-catalyzed enantioselective intramolecular hydroarylation of allylic aryl ethers devoid of a directing group on the aryl group†
Abstract
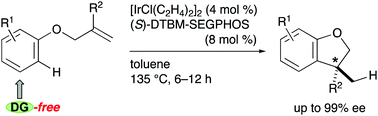
Although intramolecular hydroarylation is an attractive transformation of allylic aryl ethers, it has suffered from narrow substrate scope. We herein describe Ir/(S)-DTBM-SEGPHOS-catalyzed intramolecular hydroarylation of allylic aryl ethers. The reaction eliminates the structural requirement from the aryl group, affording 2,3-dihydrobenzofurans bearing a stereogenic carbon center at the C3 position with up to 99% enantiomeric excess.


 Please wait while we load your content...
Please wait while we load your content...