Diastereo- and enantioselective rhodium(iii)-catalyzed reductive cyclization of cyclohexadienone-containing 1,6-dienes†
Abstract
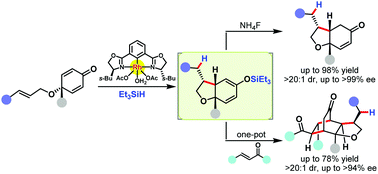
A diastereo- and enantioselective rhodium(III)-catalyzed reductive cyclization of cyclohexadienone-tethered terminal alkenes and (E)-1,2-disubstituted alkenes (1,6-dienes) is reported, providing cis-bicyclic products bearing three contiguous stereocenters with good yields and high diastereo- and enantioselectivities. The kinetic resolution of the racemic precursor is also achieved with good efficiency. Moreover, a subgram-scale experiment, several transformations of the cyclization product, and one-pot preparation of bridged polycyclic frameworks are presented.


 Please wait while we load your content...
Please wait while we load your content...