All-carbon phosphoranes via difluorocarbene trapping†
Abstract
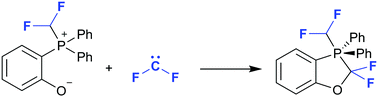
A reaction of intramolecularly disposed phosphonium and phenoxide (or thiophenoxide) fragments with difluorocarbene affording all-carbon λ5-phosphoranes is described. The presence of electron-withdrawing CHF2-groups at the phosphonium center is important for the phosphorane formation. In a phosphorane, both phenyl groups located in the equatorial positions undergo 1,2-P,C migration under thermal conditions.


 Please wait while we load your content...
Please wait while we load your content...