Tuning the P–C dative/covalent bond formation in R3P–C60 complexes by changing the R group†
Abstract
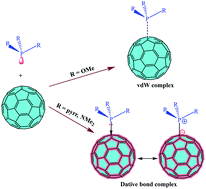
The P–C dative/covalent bonds formed in R3P–C60 complexes (R = OCH3, N(CH3)2, NC4H8) have been affected by the nature of the R group. The highest stabilisation (18.7 kcal mol−1) has been found in the last system. The contribution of dispersion energies in the stabilisation also varies depending on the R group. The nature of the P→C bond has been characterised using state-of-the-art quantum-chemical techniques including NBO, AIM and ELF. The P→C dative bond is significantly different from the prototype dative bonds appearing in H3N→BH3 as well as in the fullerene – secondary-amine complexes previously studied by us. The findings obtained through electron structure theory have been supported by 10 ps DFT-D MD simulations.


 Please wait while we load your content...
Please wait while we load your content...