A study on regulating the conjugate position of NLO chromophores for reducing the dipole moment and enhancing the electro-optic activities of organic materials
Abstract
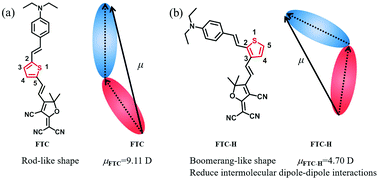
In order to improve the first-order hyperpolarizability (β) of the chromophore and transform it into a high macroscopic electro-optic activity, a series of novel second-order nonlinear optical chromophores with different push–pull electron groups introduced on the thiophene π-conjugate bridge for tuning the shape and dipole moment (μ) of chromophores were designed and synthesized. These chromophores are based on the same thiophene π-conjugated bridge, where the donor (N,N-diethylaniline) and acceptor (2-(3-cyano-4,5,5-trimethylfuran-2(5H)-ylidene)malononitrile or malononitrile) are linked to positions 2 and 3 of thiophene, respectively, affording a boomerang-like shape instead of a rod-like shape. Besides, an electron-poor group, Br (bromine atom), or an electron-rich group, DEA (N,N-diethylaniline), as an auxiliary acceptor or donor are linked to position 5 of thiophene. In addition, all chromophores showed good thermal stability as per the results from the DSC and TGA analysis. Through UV-vis analysis and DFT calculation, it has been concluded that chromophores with additional electron-rich groups as auxiliary donors display better intermolecular charge-transfer (ICT) absorption and lower HOMO–LUMO energy gaps (ΔE). Furthermore, the boomerang-like chromophore with the same push–pull structure shows a smaller dipole moment (μ) and β value than the traditional FTC. The poling results of guest–host EO polymers FTC/APC, FTC-H/APC, FTC-Br/APC and FTC-DEA/APC with the same number density afford r33 values of 17 pm V−1, 11 pm V−1, 10 pm V−1 and 25 pm V−1, respectively. Although the β value of FTC-DEA is smaller than that of FTC, the r33 value of FTC-DEA (25 pm V−1) is 47% greater than that of FTC (17 pm V−1) under the same number density. Hence, the above-mentioned results indicated that regulating the conjugate position of chromophores can efficiently decrease the dipole moment of the chromophores, weakening the dipole–dipole interactions and thereby enhancing the macroscopic electro-optical activity of poled polymers. These results indicate the potential application of these novel chromophores in electro-optical devices.


 Please wait while we load your content...
Please wait while we load your content...