Na2Fe2OS2, a new earth abundant oxysulphide cathode material for Na-ion batteries†
Abstract
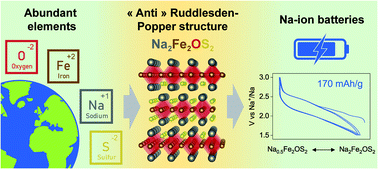
Multiple anion materials are of particular interest for the discovery of new crystal structures and offer an original way to modulate physical properties, including energy storage materials with enhanced performances. Through careful synthesis optimization, a new Na2Fe2OS2 phase was prepared by two different routes: high temperature solid-state synthesis and simple mechanochemical synthesis. The long-range and local structure of Na2Fe2OS2 was studied by Rietveld refinement of neutron and X-ray diffraction data combined with EXAFS data refinement. The phase comprises an amorphous and a crystalline part which has an anti-K2NiF4 structure, corresponding to the n = 1 member of the homologous anti-Ruddlesden–Popper [AX][ABX3]n series. Its electrochemical properties as a cathode material were studied in Na half cells and Na-ion full cells, revealing that the material becomes fully amorphous upon initial desodiation to Na0.5Fe2OS2, but maintains a reversible capacity of 135 mA h g−1 in full cells where up to 1.2 Na+ can be reversibly extracted and reinserted when compensating for the Na lost in SEI formation. The stability of the pristine material and its structural evolution upon charging are discussed, paving the way for further optimization of this material. Being composed exclusively of earth-abundant elements and stable under dry air, Na2Fe2OS2 perfectly illustrates the great opportunity of multiple anion chemistry to explore new structure types and develop better energy storage systems.


 Please wait while we load your content...
Please wait while we load your content...