Tuning proton dissociation energy in proton carrier doped 2D covalent organic frameworks for anhydrous proton conduction at elevated temperature†
Abstract
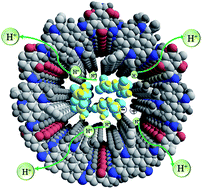
A theoretical and experimental study gives insights into the change of proton dissociation energy of anhydrous proton carriers (phosphoric acid and 1,2,4-triazole) doped in 2D covalent organic frameworks (COFs) with neutral, polar, Lewis base and positively charged sites in their 1D channels. The dielectric properties of proton carrier incorporated COFs were investigated to determine the formation of nanoscale ionic phases in COFs' channels. The proton carrier doped cationic COF exhibits a much higher dielectric constant in the frequency range of 103 Hz to 107 Hz than other doped COFs, which may arise from the formation of ethidium-biphosphate or ethidium-triazole ion-pairs in charged COF channels. The ion-pairs lined along cationic COFs' channels produce an enhanced proton dissociation degree coupled with a high dielectric response, leading to a new proton conductivity record (2.77 × 10−2 S cm−1) set by the cationic COF among all reported porous materials under anhydrous conditions and elevated temperatures.


 Please wait while we load your content...
Please wait while we load your content...