Bimetallic Ni–Pt nanoparticles immobilized on mesoporous N-doped carbon as a highly efficient catalyst for complete hydrogen evolution from hydrazine borane†
Abstract
In this work, mesoporous N-doped carbon (MNC) materials have been successfully prepared by a nanocasting route using SBA-15 as the template and ethylenediamine (EDA) and carbon tetrachloride (CTC) as carbon nitride precursors. The specific surface area and pore size of the MNC, as well as the C/N content are strongly dependent on the pyrolysis temperature. The as-obtained MNC-supported NiPt nanoparticles (NPs) were used as efficient catalysts toward complete dehydrogenation of hydrazine borane (HB) in alkaline solution at room temperature. Among all the catalysts investigated, the Ni60Pt40/MNC-800 nanocomposites (NCs) show the highest catalytic activity and 100% hydrogen selectivity. The total turnover frequency (TOF) of Ni60Pt40/MNC-800 NCs reached 1111 h−1 at room temperature, the highest activity among all catalysts reported to date. The superior catalytic properties were possibly a result of the electronic interaction between Ni and Pt, the synergistic effects of the NiPt NPs and MNC support, the ordered pore structure for free mass transfer and the small-sized metal NPs.
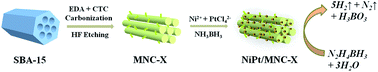


 Please wait while we load your content...
Please wait while we load your content...