NixFe1−xB nanoparticle self-modified nanosheets as efficient bifunctional electrocatalysts for water splitting: experiments and theories†
Abstract
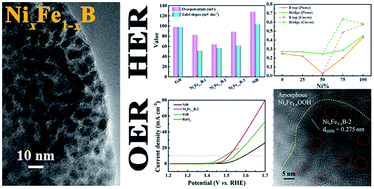
Developing highly efficient, stable, and inexpensive electrocatalysts for both the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER) is the premise of achieving large-scale water splitting. Here, NixFe1−xB prepared by a facile borothermal reduction in molten salt is a robust bifunctional electrocatalyst for overall water splitting. The NixFe1−xB electrocatalyst achieves a current density of 10 mA cm−2 at overpotentials of 63.5 mV for the HER and 282 mV for the OER in 1 M KOH electrolyte, respectively. Density functional theory (DFT) calculations suggest that the NixFe1−xB possesses a lower absolute value of H* adsorption free energy (|ΔGH*|) than the primitive FeB and NiB, which is beneficial to improve the HER activity. Two identical NixFe1−xB electrodes are combined to form an alkaline electrolyzer, which achieves a current density of 10 mA cm−2 at 1.57 V for overall water splitting. This work provides a strategy to develop highly efficient transition metal boride catalysts for overall water splitting.


 Please wait while we load your content...
Please wait while we load your content...