Black phosphorus synthesized by solvothermal reaction from red phosphorus and its catalytic activity for water splitting†
Abstract
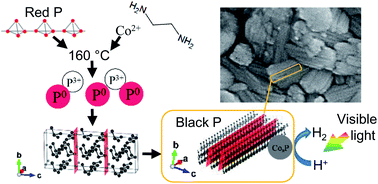
We have succeeded in synthesizing black phosphorus (BP) by a one-pot solvothermal reaction of red phosphorus (RP) and ethylenediamine (ED) used as a solvent. To examine the reaction mechanism, we have investigated the influence of the synthesis conditions on BP, and the valence of dissolved phosphorus in the solvent after the reaction. P0 species and P3+ like species are very likely dissolved in ED as intermediates for BP production. Optimizing the reaction conditions, i.e., temperature, the charging amount of RP in ED and the particle size of RP, BP was synthesized with high yield. The synthesized BP has an extra peak at 10.2° in the X-ray diffraction pattern, which was assigned to stacking faults or periodic distortion in the direction of the c axis by simulation of diffraction. The synthesized BP with a Co–P cocatalyst showed high photocatalytic activity for hydrogen evolution from methanol aqueous solution under visible light irradiation.


 Please wait while we load your content...
Please wait while we load your content...