Perovskite-supported Pt single atoms for methane activation
Abstract
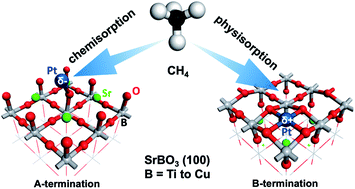
ABO3 perovskites are increasingly being explored as catalysts, but it is unclear how they behave as supports for single atoms and how the subsequent single-atom catalysts can be employed for important reactions such as methane activation. Here we examine the stability of Pt single atoms (Pt1) on the commonly exposed (100) surfaces of SrBO3 perovskites (B = 3d transition metals) and their methane-adsorption properties by first principles density functional theory. We find that the stability and charge state of Pt1 on the SrBO3(100) surfaces are termination-sensitive. Due to polar compensation, Pt1 is negatively charged on the A termination but positively charged on the B termination. This charge state greatly impacts methane adsorption: negatively charged Pt1 on the A-termination chemisorbs methane (in some cases, dissociatively), but positively charged Pt1 on the B-termination adsorbs methane physically. Analysis of the density of states of the negatively charged Pt1 reveals that its sp states are key to methane chemisorption and C–H activation. Our work shows that polar compensation on the perovskite surfaces can be used to tune the charge state of a single atom for methane chemisorption and C–H activation.


 Please wait while we load your content...
Please wait while we load your content...