Low electronegativity Mn bulk doping intensifies charge storage of Ni2P redox shuttle for membrane-free water electrolysis†
Abstract
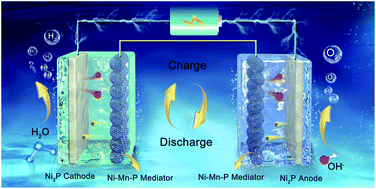
A high efficiency electrochemical energy storage and high areal capacitance solid-state mediator can act as an energy vector to solve the key problem in mixtures of H2 and O2 in electrically driven water splitting architectures. It is believed that introducing foreign metal atoms into a monometallic compound could promote the energy storage capacity of the mediator. Herein, based on the theoretical prediction that partial substitution of low electronegativity Mn in the Ni2P structure could enhance the local charge density around Ni and lower the rate-determining Gibbs free energy difference (ΔGOOH–ΔGO) compared to Fe substitution or the pure compound, a supercapacitor-type transition metal phosphide (TMP) mediator Ni–Mn–P with an areal capacitance of 12.25 F cm−1 is fabricated. Benefitting from the enhanced energy storage activity and high exposure of active sites, the charge storage in the H2 generation step in a 1 cm2 Ni–Mn–P mediator electrode when coupled with Ni2P/NF bifunctional electrodes maintains >500 s O2 evolution (10 mA), ∼4 times longer than that of an unsubstituted Ni–P mediator. In addition, the superior stability for >20 000 s cycle operation at 20 mA further demonstrates its operational flexibility and potential for practical application.


 Please wait while we load your content...
Please wait while we load your content...