Oscillatory structural forces between charged interfaces in solutions of oppositely charged polyelectrolytes†
Abstract
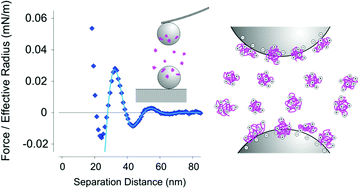
Forces between negatively charged micron-sized silica particles were measured in aqueous solutions of cationic polyelectrolytes with an atomic force microscope (AFM). In these oppositely charged systems, damped oscillatory force profiles were systematically observed in systems at higher polyelectrolyte concentrations, typically around few g L−1. The wavelength of these oscillations is decreasing with increasing concentration. When the wavelength and concentration are normalized with the cross-over concentration, universal power-law dependence is found. Thereby, the corresponding scaling exponent changes from 1/3 in the dilute regime to 1/2 in the semi-dilute regime. This dependence is the same as in the like-charged systems, which were described in the literature earlier. This common behavior suggests that these oscillatory forces are related to the structuring of the polyelectrolyte solutions. The reason that the oppositely charged systems behave similarly to like-charged ones is that the former systems undergo a charge reversal due to the adsorption of the polyelectrolytes to the oppositely charged surface, whereby sufficiently homogeneous adsorbed layers are being formed. The main finding of the present study is that at higher polyelectrolyte concentrations such oscillatory forces are the rule, including the oppositely charged ones.


 Please wait while we load your content...
Please wait while we load your content...