Liquid–polymer triboelectricity: chemical mechanisms in the contact electrification process†
Abstract
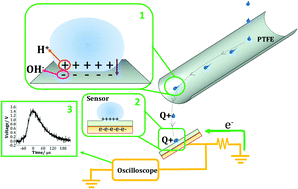
Liquid–polymer contact electrification between sliding water drops and the surface of polytetrafluoroethylene (PTFE) was studied as a function of the pH and ionic strength of the drop as well as ambient relative humidity (RH). The PTFE surface was characterized by using SEM, water-contact-angle measurements, FTIR spectroscopy, XPS, and Raman spectroscopy. The charge acquired by the drops was calculated by detecting the transient voltage induced on a specifically designed capacitive sensor. It is shown that water drops become positively charged at pH > pHzch (pHzch being the zero charge point of the polymer) while they become negatively charged for pH < pHzch. The addition of non-hydrolysable salts (NaCl or CaCl2) to water decreases the electrical charge induced in the drop. The charge also decreases with increasing RH. These results suggest proton or hydroxyl transfer from the liquid to the hydrophobic polymer surface. A proposed thermodynamic model for the ion transfer process allows explaining the observed effects of RH, pH and ionic strength.


 Please wait while we load your content...
Please wait while we load your content...