Molecularly imprinted peptide-based enzyme mimics with enhanced activity and specificity†
Abstract
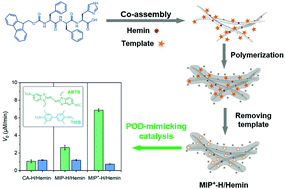
We herein report the construction of peroxidase (POD)-mimicking catalysts based on the strategy of peptide assembly and molecular imprinting. Upon co-assembly of Fmoc-FFH and Hemin, we firstly fabricated CA-H/Hemin which displayed POD-like catalytic activity and showed a 21-fold rate acceleration in the oxidation of 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) compared to the uncatalyzed reaction. Then, upon combining CA-H/Hemin with the ABTS-imprinted polymer, the obtained imprinted catalyst (MIP-H/Hemin) showed 52-fold acceleration due to the enhanced re-binding toward ABTS. Moreover, by introducing cationic monomers, a 137-fold rate enhancement was further achieved for the positively charged imprinted catalyst (MIP+-H/Hemin), from the synergistic effect of molecular imprinting and electrostatic attraction. Remarkably, by comparing the catalytic activity of these POD mimics towards ABTS and 3,3′,5,5′-tetramethylbenzidine (TMB), we also highlighted the substrate specificity of MIP-H/Hemin and MIP+-H/Hemin toward ABTS. This study provides a promising approach to improve the catalytic activity and specificity of peptide-based enzyme mimics.


 Please wait while we load your content...
Please wait while we load your content...