Enhanced electrocatalytic and supercapacitive performance using the synergistic effect of defect-rich N/S co-doped hierarchical porous carbon†
Abstract
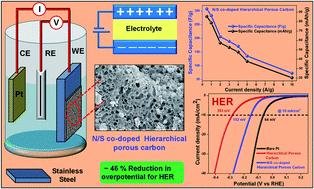
Template free N/S co-doped hierarchical porous carbon has been synthesized using biomass alginic acid and organic moiety sulfanilic acid as precursors via rapid thermal decomposition, followed by KOH activation and acid etching. The chemical composition and morphological features of the synthesized materials are well characterized using X-ray photoelectron spectroscopy and scanning as well as transmission electron microscopy, respectively. Hierarchical pristine and N/S co-doped carbon materials are utilized as model candidates for electrocatalytic water reduction and supercapacitor applications. N/S co-doped hierarchical porous carbon shows better water reduction activity having a low overpotential value (∼152 mV at 10 mA cm−2 current density) and Tafel slope (∼59 mV dec−1). Moreover, the N/S co-doped hierarchical porous carbon-based active electrode delivers a remarkable specific capacitance of ∼307 F g−1 (∼85.25 mA h g−1) at 0.50 A g−1 current density, estimated from the galvanostatic charging–discharging curve. The enhanced electrocatalytic and capacitive activity of N/S co-doped hierarchical porous carbon are associated with the large concentration of surface-active sites with micropore-/mesopore-rich nanoarchitectures, improved electrical conductivity, large interlayer distance for better electrode/electrolyte interaction, and lower charge transfer resistance. The N/S co-doped hierarchical porous carbon-based electrode shows superior cycling stability and reversibility without a significant reduction in specific capacity even after 1000 cycles.


 Please wait while we load your content...
Please wait while we load your content...