Selective hydrogenation of furfural for high-value chemicals: effect of catalysts and temperature†
Abstract
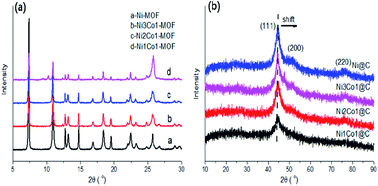
Transformation of furfural (FFA), a typical representative of furan platform chemicals derived from the acid hydrolysis of hemicellulose to the high value chemicals tetrahydrofurfuryl alcohol (THFOL) and cyclopentanol (CPL), has drawn great attention. In this study, we report an efficient NiCo bimetallic catalyst with highly dispersed NiCo-based metals on a porous carbon matrix for FFA hydrogenation. For different FFA conversion reactions of the bimetallic catalysts, the tetrahydrofurfuryl (THFOL) yield was up to 95% over the Ni3Co1@C catalyst at 80 °C. Furthermore, cyclopentanol (CPL) could also be obtained with a yield of 95% with Ni1Co1@C as the catalyst at 160 °C in an aqueous medium. The detailed physicochemical characterization was carried out by means of XRD, SEM, BET, ICP, XPS, NH3-TPD and Raman analysis. With the addition of Co in the bimetallic catalysts, the average particle size decreased obviously to around 5.7 nm in NixCoy/C catalysts with different Ni/Co ratios, which increased the dispersion and improved the catalytic activity of FFA hydrogenation. The NixCoy@C catalysts could be recovered and efficiently applied in the next run for four consecutive recycling tests in FFA hydrogenation to the corresponding target products under different reaction conditions. The results suggested that the NixCoy@C catalyst appeared to increasingly favor the formation of Ni–Co alloys and suggested a metallic active site in FFA hydrogenation with the addition of the Co element. Mechanistic study indicated that temperature was a key factor contributing to the formation of different desired products (THFOL and CPL). Furthermore, water was another essential factor, which was responsible for the arrangement of the furan compound.


 Please wait while we load your content...
Please wait while we load your content...