Co-production of pure hydrogen, carbon dioxide and nitrogen in a 10 kW fixed-bed chemical looping system
Abstract
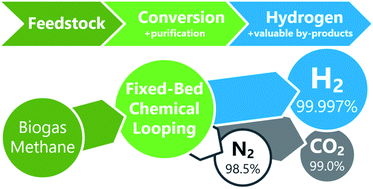
The transition of our current carbon-based economy towards a sustainable energy system poses major challenges for all stakeholders. Harmful carbon dioxide emissions have to be substantially decreased and even negative emissions are mandatory to avoid a global mean temperature rise above 2 °C unless stringent regulatory measures are taken within the next decade. Chemical looping is a promising method to sequestrate pure carbon dioxide from fossil and renewable energy resources within the framework of carbon capture and storage (CCS) or utilization (CCU) technologies. The presented study demonstrates the generation of high-purity hydrogen exceeding 99.997% as a zero-emission energy carrier with the inherent co-generation of pure carbon dioxide (99%) and nitrogen (98.5%) in the largest fixed-bed chemical looping research system worldwide. The feedstock utilization of up to 60% in the context of pure hydrogen generation is highly competitive compared to other systems for decentralized hydrogen generation with the benefit of inherent carbon dioxide sequestration. The use of renewable primary energy sources as biogas qualifies the process as a negative emission technology (NET) if carbon dioxide is appropriately utilized.


 Please wait while we load your content...
Please wait while we load your content...