Catalytic depolymerization of alkali lignin in ionic liquids on Pt-supported La2O3–SO42−/ZrO2 catalysts†
Abstract
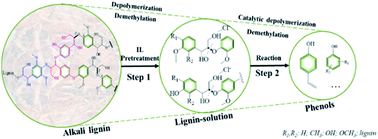
A series of PtLaχ/SO42−/ZrO2 catalysts, where χ is the atomic ratio of La to Pt of 1, 3 and 6, were prepared and used for the catalytic depolymerization of alkali lignin with ionic liquids in a continuous flow fixed-bed reaction system. Compared to the SO42−/ZrO2 catalyst, the introduction of Pt and La2O3 significantly enhanced the catalytic activity in alkali lignin depolymerization. According to the characterization results of the catalysts obtained from N2-physisorption, FT-IR spectra of CO or pyridine adsorption, and XPS and TEM analysis, the introduction of Pt and La2O3 resulted in the highly covalent character of sulfate species on the catalyst surface and strong interactions between Pt and La2O3, which led to an increase of the number of Lewis acid sites for PtLaχ/SZ catalysts. The PtLa3/SO42−/ZrO2 catalyst showed the highest yield of phenolic compounds (28.7%) at 210 °C. Furthermore, based on the results of two model compounds of lignin in pretreatment and reaction units, the synergistic effect of ionic liquids and catalysts was also elucidated and a hypothetical reaction pathway of lignin depolymerization was elucidated.


 Please wait while we load your content...
Please wait while we load your content...