The sporothriolides. A new biosynthetic family of fungal secondary metabolites†
Abstract
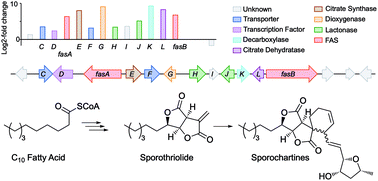
The biosynthetic gene cluster of the antifungal metabolite sporothriolide 1 was identified from three producing ascomycetes: Hypomontagnella monticulosa MUCL 54604, H. spongiphila CLL 205 and H. submonticulosa DAOMC 242471. A transformation protocol was established, and genes encoding a fatty acid synthase subunit and a citrate synthase were simultaneously knocked out which led to loss of sporothriolide and sporochartine production. In vitro reactions showed that the sporochartines are derived from non-enzymatic Diels–Alder cycloaddition of 1 and trienylfuranol A 7 during the fermentation and extraction process. Heterologous expression of the spo genes in Aspergillus oryzae then led to the production of intermediates and shunts and delineation of a new fungal biosynthetic pathway originating in fatty acid biosynthesis. Finally, a hydrolase was revealed by in vitro studies likely contributing towards self-resistance of the producer organism.


 Please wait while we load your content...
Please wait while we load your content...