Nature of the copper-nitrosyl intermediates of copper nitrite reductases during catalysis†
Abstract
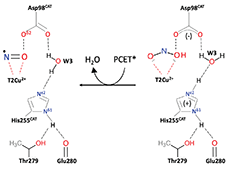
The design and synthesis of copper complexes that can reduce nitrite to NO has attracted considerable interest. They have been guided by the structural information on the catalytic Cu centre of the widespread enzymes Cu nitrite reductases but the chemically novel side-on binding of NO observed in all crystallographic studies of these enzymes has been questioned in terms of its functional relevance. We show conversion of NO2− to NO in the crystal maintained at 170 K and present ‘molecular movies’ defining events during enzyme turnover including the formation of side-on Cu-NO intermediate. DFT modelling suggests that both true {CuNO}11 and formal {CuNO}10 states may occur as side-on forms in an enzymatic active site with the stability of the {CuNO}10 side-on form governed by the protonation state of the histidine ligands. Formation of a copper-nitrosyl intermediate thus needs to be accommodated in future design templates for functional synthetic Cu-NiR complexes.


 Please wait while we load your content...
Please wait while we load your content...