Controlled synthesis of organic two-dimensional nanostructures via reaction-driven, cooperative supramolecular polymerization†
Abstract
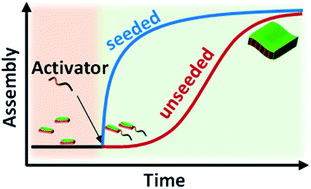
The bottom-up approach of supramolecular polymerization is an effective synthetic method for functional organic nanostructures. However, the uncontrolled growth and polydisperse structural outcome often lead to low functional efficiency. Thus, precise control over the structural characteristics of supramolecular polymers is the current scientific hurdle. Research so far has tended to focus on systems with inherent kinetic control by the presence of metastable state monomers either through conformational molecular design or by exploring pathway complexity. The need of the hour is to create generic strategies for dormant states of monomers that can be extended to different molecules and various structural organizations and dimensions. Here we venture to demonstrate chemical reaction-driven cooperative supramolecular polymerization as an alternative strategy for the controlled synthesis of organic two-dimensional nanostructures. In our approach, the dynamic imine bond is exploited to convert a non-assembling dormant monomer to an activated amphiphilic structure in a kinetically controlled manner. The chemical reaction governed retarded nucleation–elongation growth provides control over dispersity and size.
- This article is part of the themed collections: Celebrating the Chemical Sciences in India - Leaders in the Field Symposium 2021, Celebrating the Chemical Sciences in India - Leaders in the Field Symposium 2020, Celebrating 10 years of Chemical Science and Celebrating the Chemical Science in India - Leaders in the Field Symposium


 Please wait while we load your content...
Please wait while we load your content...