The influences of carbon donor ligands on biomimetic multi-iron complexes for N2 reduction†
Abstract
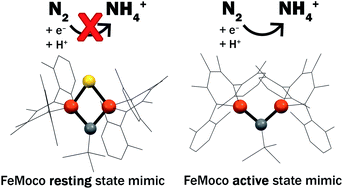
The active site clusters of nitrogenase enzymes possess the only examples of carbides in biology. These are the only biological FeS clusters that are capable of reducing N2 to NH4+, implicating the central carbon and its interaction with Fe as important in the mechanism of N2 reduction. This biological question motivates study of the influence of carbon donors on the electronic structure and reactivity of unsaturated, high-spin iron centers. Here, we present functional and structural models that test the impacts of carbon donors and sulfide donors in simpler iron compounds. We report the first example of a diiron complex that is bridged by an alkylidene and a sulfide, which serves as a high-fidelity structural and spectroscopic model of a two-iron portion of the active-site cluster (FeMoco) in the resting state of Mo-nitrogenase. The model complexes have antiferromagnetically coupled pairs of high-spin iron centers, and sulfur K-edge X-ray absorption spectroscopy shows comparable covalency of the sulfide for C and S bridged species. The sulfur-bridged compound does not interact with N2 even upon reduction, but upon removal of the sulfide it becomes capable of reducing N2 to NH4+ with the addition of protons and electrons. This provides synthetic support for sulfide extrusion in the activation of nitrogenase cofactors.
- This article is part of the themed collection: Celebrating 10 years of Chemical Science


 Please wait while we load your content...
Please wait while we load your content...