Synthesis of side-chain regioregular and main-chain alternating poly(bichalcogenophene)s and an ABC-type periodic poly(terchalcogenophene)†
Abstract
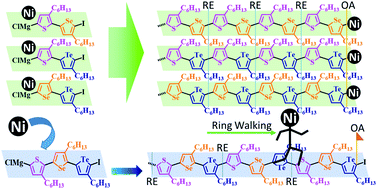
Three unsymmetrical diiodobichalcogenophenes SSeI2, STeI2, and SeTeI2 and a diiodoterchalcogenophene SSeTeI2 were prepared. Grignard metathesis of SSeI2, STeI2, SeTeI2, and SSeTeI2 occurred regioselectively at the lighter chalcogenophene site because of its relatively lower electron density and less steric bulk. Nickel-catalyzed Kumada catalyst-transfer polycondensation of these Mg species provided a new class of side-chain regioregular and main-chain AB-type alternating poly(bichalcogenophene)s—PSSe, PSTe, and PSeTe—through a chain-growth mechanism. The ring-walking of the Ni catalyst from the lighter to the heavier chalcogenophene facilitated subsequent oxidative addition, thereby suppressing the possibility of chain-transfer or chain-termination. More significantly, the Ni catalyst could walk over the distance of three rings (ca. 1 nm)—from a thiophene unit via a selenophene unit to a tellurophene unit—to form PSSeTe, the first ABC-type regioregular and periodic poly(terchalcogenophene) comprising three different types of 3-hexylchalcogenophenes.


 Please wait while we load your content...
Please wait while we load your content...