Facilitating the reduction of V–O bonds on VOx/ZrO2 catalysts for non-oxidative propane dehydrogenation†
Abstract
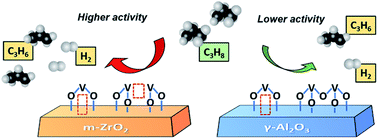
Supported vanadium oxide is a promising catalyst in propane dehydrogenation due to its competitive performance and low cost. Nevertheless, it remains a grand challenge to understand the structure–performance correlation due to the structural complexity of VOx-based catalysts in a reduced state. This paper describes the structure and catalytic properties of the VOx/ZrO2 catalyst. When using ZrO2 as the support, the catalyst shows six times higher turnover frequency (TOF) than using commercial γ-Al2O3. Combining H2-temperature programmed reduction, in situ Raman spectroscopy, X-ray photoelectron spectroscopy and theoretical studies, we find that the interaction between VOx and ZrO2 can facilitate the reduction of V–O bonds, including V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, V–O–V and V–O–Zr. The promoting effect could be attributed to the formation of low coordinated V species in VOx/ZrO2 which is more active in C–H activation. Our work provides a new insight into understanding the structure–performance correlation in VOx-based catalysts for non-oxidative propane dehydrogenation.
O, V–O–V and V–O–Zr. The promoting effect could be attributed to the formation of low coordinated V species in VOx/ZrO2 which is more active in C–H activation. Our work provides a new insight into understanding the structure–performance correlation in VOx-based catalysts for non-oxidative propane dehydrogenation.
- This article is part of the themed collection: Celebrating the Chemical Sciences in India - Leaders in the Field Symposium 2021


 Please wait while we load your content...
Please wait while we load your content...