New reductive rearrangement of N-arylindoles triggered by the Grubbs–Stoltz reagent Et3SiH/KOtBu†
Abstract
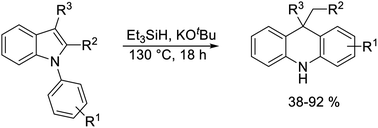
N-Arylindoles are transformed into dihydroacridines in a new type of rearrangement, through heating with triethylsilane and potassium tert-butoxide. Studies indicate that the pathway involves (i) the formation of indole radical anions followed by fragmentation of the indole C2–N bond, and (ii) a ring-closing reaction that follows a potassium-ion dependent hydrogen atom transfer step. Unexpected behaviors of ‘radical-trap’ substrates prove very helpful in framing the proposed mechanism.


 Please wait while we load your content...
Please wait while we load your content...